Featured Story
![]()
March 2006
Smart Weapons
![]()
With an arsenal of quills and chemicals, the porcupine mounts
one of nature’s most robust defenses against predators.
![]()
By Uldis Roze
IT IS A CLEAR, midsummer midnight in the Catskill Mountains of upstate New York, and I’m trying to capture Loretta, an adult female porcupine. In preparation, I’m wearing heavy vinyl gloves to protect myself from Loretta’s bristling armor of quills. I plan to scoop her up and place her temporarily into a snug, three-gallon picnic cooler, then make some measurements and observations for my research on the social structure of her species.
|
Porcupines, for the most part, have a sweet and trusting disposition that comes only to those who have little reason to be afraid. Of course, quills are the animal’s best-known defense. Each quill is between one-half and four inches long, with one-way barbs for burrowing into the victim’s body and an antibiotic coating to limit the damage if the porcupine quills itself. The quills number in the tens of thousands and cover every inch of its body, with the exception of its face, belly, and the undersides of its limbs and tail.
But there is more to a sense of security than merely possessing an advanced weapon. If your enemies attack you, you may win in the end, but you still risk being injured in the process. To avoid a fight at all, you have to deter an attack with warnings. Your enemies have to realize that you possess your weapon, and be reminded, in no uncertain terms, that if you’re attacked, you will use it. Thus porcupines broadcast a distinct, pungent warning odor when their quills are erected. Furthermore, the quills contain a fluorescent material that brightens the quills at night, when the porcupine is most likely to meet predators. Those evolutionary adaptations ensure a safe infancy for porcupine offspring and relatively long life for the adult—one radio-tagged female lived in my Catskills study area for twenty-one years.
I strip off the quill-perforated glove with my teeth, and finish the capture barehanded. I clap the cooler’s lid over Loretta to immobilize her dangerous tail and lower back. Little drops of blood speckle my hand and fingers. But I have been lucky—none of the quill tips have broken off to travel deeper into my body. I weigh my prickly friend, note that she is lactating, and then let her go. She moves off briskly to her baby in the woods.
But Loretta has left something of herself behind—a small forest of quills embedded in my rubberized glove. To use the glove again, I must pull out all the quills. But when I start pulling, I am struck by how firmly the quills are anchored in the glove. So instead of just finishing the job with fingers or long-nose pliers, I decide to measure how much force is needed to withdraw each quill.
I have an accurate spring balance, with a maximum capacity of 10.5 ounces. All I have to do is attach an alligator clip to the spring and grip each quill with the clip while I give a pull. I tally eighty-four quills, and I measure 6.7 ounces of force per quill, on average, to extract each one of them. In fact, my result is a gross underestimate. Twenty of the quills in my glove take more pull than the balance can register. In a later experiment I discover the extraction tension for individual, well-rooted quills can be twenty-five times higher than my first calculation suggests, or in excess of ten pounds apiece!
|
Even if the extraction force were “only” 6.7 ounces per quill, extracting all eighty-four quills at once would take a pull of more than thirty-five pounds. That is well above Loretta’s body weight—thirteen pounds—and far more force than she could conceivably exert on her own, especially considering that porcupines have relatively little muscle compared with other mammals.
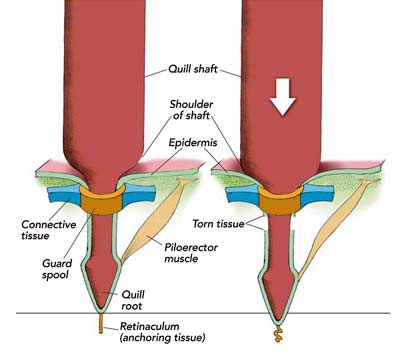
So how did Loretta separate herself from the glove? Not by pulling her quills out of it. Instead, she shed them from her skin. Does that solve the paradox? It might if eighty-four quills could be removed from Loretta’s skin with a force roughly equal to her weight—about two and a half ounces per quill. I do the obvious experiment. I anesthetize Loretta and seven other porcupines with a quick-acting drug, and measure the withdrawal tension of a few of the animals’ quills. The average quill-withdrawal tension is 3.2 ounces per quill, still too much for a little animal to disengage quickly from her target. In other words, when Loretta struck my glove, she should have remained stuck to it, tied down like a bristly Gulliver by multiple tiny bonds.The fact that Loretta was able to break her eighty-four connections in a flash suggests something is wrong with my analysis. David M. Chapman, a histologist at Lakehead University in Thunder Bay, Ontario, who has studied porcupine skin and quill follicles microscopically, offers an alternate explanation: the force needed to separate a quill follicle from the skin of the porcupine may drop after the quill has been driven into an adversary. Consider one of the quills stuck in my rubberized glove. When the quill tip struck the glove, an equal and opposite reactive force drove the quill root deeper into the skin of the porcupine. The inward push was probably violent enough to break some of the attachments between the base of the quill and its surrounding tissues. As a result, less force would have been needed to separate the quill from the porcupine’s body.
How to test the hypothesis? Pulling quills from a porcupine on the defensive is difficult and dangerous business. Chapman suggests an elegant way around the problem. Strike the back of an aroused porcupine with a block of something light and penetrable, such as cork or Styrofoam, and leave it in place. Then anesthetize the animal, separate the block from the animal by cutting off the tips of the quills embedded in the block, and measure the force needed to pull the quills with the cut-off tips out of the animal’s skin.
I try the technique on a female I have named Heart. The results are clear-cut: it takes, on average, only 1.9 ounces per quill to pull the six struck quills out of the animal. By contrast, it takes 3.4 ounces per quill to pull six undisturbed quills from the same area on the porcupine. Experiments with other porcupines confirm that the tension required to extract a quill from a porcupine is reduced by about 40 percent if the quill is first driven into the porcupine’s body. That’s exactly what would happen after a tail slap or other violent contact with an antagonist.
|
When the porcupine is relaxed, the guard spools move freely: if you strike the quills of an anesthetized porcupine, the spools just glide in with the impact, and the quills remain anchored in the animal as firmly as ever. (That property guarantees, for instance, that a sleeping porcupine doesn’t lose its quills if they accidentally press against a tree trunk.) When a porcupine is provoked, however, and its quills are erect, the guard spools are held in place by taut connective tissue in the skin. If a strong downward force is applied to the quill’s shaft, it drives the quill root deeper into the porcupine and shears it from the surrounding tissue. The animal can then readily shed the quill and escape its injured adversary [see illustration left].
But battles with predators do carry the risk of injury to the porcupine. I once examined the skull of a porcupine at the University of Wisconsin Zoological Museum in Madison that bore silent witness to a violent encounter. The skull dates from the 1890s, when wolves and wolverines shared the porcupine’s habitat. The skull was indented on top and partly flattened by the jackhammer impact of a canine’s crunching down. Subsequent healing shows that the porcupine survived, yet the margin between life and death must have been thin.
To avoid such battles, the porcupine issues warnings, and its primary warning signal is olfactory. As a porcupine waits for an attack, quills erect, it pours out a wave of pungent odor to signal that its foe would do better to back off.
The odor is generated by a patch of skin called the rosette, on the porcupine’s lower back. Specialized quills growing out of the rosette help broadcast the smell. Biologists have long noted modified hairs that disseminate odors in other mammals: black-tailed deer,
 the crested rat of East Africa, several bat species, and others. Such hairs are called osmetrichia, and they differ from ordinary hairs in having increased surface area and in their ability to stand erect when the animal is on alert. The greater surface area holds more odorant molecules, and the erectability helps disseminate the molecules into the air. In porcupines, the barb-covered section of the quills in the rosette area is longer than it is on quills of the upper back, and the rosette barbs themselves have the greater overlap. Both effects increase the surface area of the rosette quills.
the crested rat of East Africa, several bat species, and others. Such hairs are called osmetrichia, and they differ from ordinary hairs in having increased surface area and in their ability to stand erect when the animal is on alert. The greater surface area holds more odorant molecules, and the erectability helps disseminate the molecules into the air. In porcupines, the barb-covered section of the quills in the rosette area is longer than it is on quills of the upper back, and the rosette barbs themselves have the greater overlap. Both effects increase the surface area of the rosette quills.
Just as the swat by Loretta got me hooked on how quills exit the porcupine, another nighttime encounter propelled me on the path to identifying the porcupine’s warning odor. Passing under an apple tree in the dark, I sensed an alarmed porcupine on a branch above me simply by its wave of smell. That warning smell has a penetrating quality somewhat similar to the smell of goat or perhaps an exotic cheese.
| Threatened by a predator, a porcupine (above) hunkers down with its head away from the danger, preparing to use a slap of its tail to thrust sharp-pointed quills into its enemy (dog below). To avoid a full-blown confrontation, the porcupine also emits a highly distinctive and pungent odor, warning its foe to back off. Illustrations © Alan Barke |
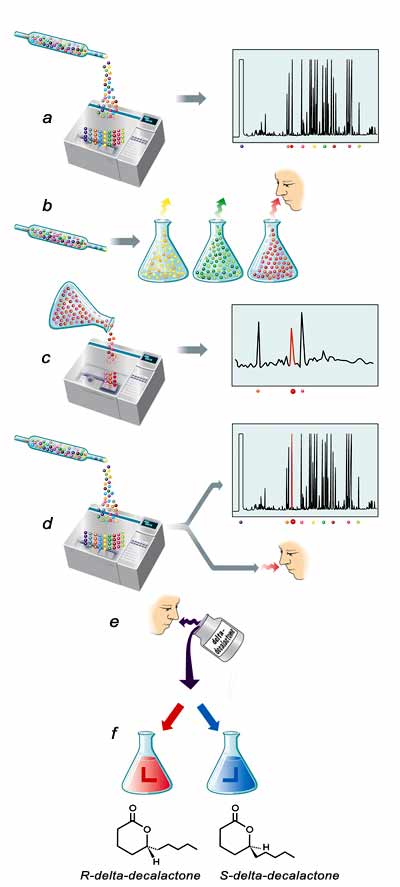
 I asked David C. Locke, a chemist at Queens College in New York City, whether he could help me identify the warning-smell molecule. David operates a gas chromatograph-mass spectrometer (GC-MS). The gas chromatograph sorts a gas mixture into its various components, according to the rate at which each component gas leaves the system. Then the mass spectrometer helps identify each component by its molecular mass and fragmentation pattern.
I asked David C. Locke, a chemist at Queens College in New York City, whether he could help me identify the warning-smell molecule. David operates a gas chromatograph-mass spectrometer (GC-MS). The gas chromatograph sorts a gas mixture into its various components, according to the rate at which each component gas leaves the system. Then the mass spectrometer helps identify each component by its molecular mass and fragmentation pattern.
Before the instrument can work its magic, however, a smell must be captured. A portable air pump draws the odor through a cartridge that contains charcoal, silica gel, or some other odor-absorbing compound, until the compound is saturated. The odor is released, or “desorbed,” by heating or adding a solvent, and passed through the GC-MS.
I set to work capturing a porcupine, securing it in a picnic cooler, then sucking air through the cooler and through a cartridge. David advised drawing the air for at least two hours to saturate the cartridge, but that’s much longer than I usually keep my guests. Besides, this porcupine has other ideas. After a quarter hour, it grows bored with its tight, dark enclosure and begins chewing the plastic walls of the cooler to get out. I hurriedly release the animal, then continue to pump air through the damaged cooler, which reeks of angry porcupine.
David desorbs the cartridge and runs it through the GC-MS. Now comes the first reality check. What the printout reveals about the cooler’s environment is not what is perceived by the human nose. Instead of the smell of angry porcupine, the GC-MS detects a jumble of thirty compounds.
|
At this point, I realize that a good part of the residual porcupine smell is coming from quills scattered on the bottom of the cooler, not from the cooler walls. That makes my job a lot simpler. I won’t need to collect odor from a living, thrashing porcupine—freshly pulled quills will do just fine.
David assigns the problem of structure determination to a talented graduate student, Guang Li, who sets competently to work. We eliminate the problem of room-air contaminants by filtering the incoming air. Guang improves the discrimination of the system until eighty-nine compounds in the porcupine odor can be chemically separated. The active principle of porcupine warning odor must be lurking somewhere among the eighty-nine peaks of Guang’s chromatogram. But which one?
Guang sets out to trap the porcupine odor in a different way. Solvents such as water or alcohol vary in polarity, or the amount by which negative electric charge is concentrated on one side of the solvent molecule and positive charge on the other. Guang knows that solvents of differing polarity extract different components of a mixture, so he washes a cartridge containing porcupine odor through three solvents of increasing polarity.
But Guang has never encountered a porcupine and doesn’t know what one smells like, so he asks me to smell the three extracts. The first vial is odorless. The second vial has an odor, but it is not porcupine-like. But the third vial, collected with the strongest solvent, strikes the nose with a strong porcupine smell. It is an eerie experience, smelling porcupine in a bottle—one has a sense of imprisoned wildness, like the unfortunate genie of Arabian folk tales. On chromatography, the third vial sorts into just three principal components. Two of them are common compounds that can be eliminated at once. The third is an unusual compound called delta-decalactone, a ring-shaped molecule with ten carbon atoms, two oxygens, and eighteen hydrogens.
To confirm this molecule is the active element of the warning odor, Guang sets up an elegant experiment. He sends the contents of an odor cartridge impregnated with porcupine odor through a gas chromatograph. Then he splits the instrument’s output—the usual eighty-nine components—into two parts. One part goes to a strip-chart recorder, which generates the familiar pattern of peaks and valleys of porcupine odor. The other part goes to a biological detector—the human nose. Locke, Guang, and I all donate our noses for detector duty. (By this time Guang has accompanied me on a Catskill visit to catch and smell a porcupine, and so recognizes its unique odor.) We take turns going off nose duty so that one of us can annotate the strip-chart recorder.
Then it happens. “Porcupine!” I cry out, and Guang marks the spot on the strip chart. The odor builds, incredibly strong because the vapors are heated, with the signature of pure porcupine. Then it ends, and there is olfactory silence. The peak that Guang has marked on the strip chart is delta-decalactone.
Only one small step is left: to cross-check the odor against commercial delta-decalactone. The small, brown bottle arrives. I unscrew the cap and sniff. Oh, no, there is a strong smell of coconut, not the expected porcupine. Something is terribly wrong with our hypothesis.
In the excitement of assigning a name to the porcupine odor, we had forgotten that delta-decalactone is the name for two closely related compounds. The two are optical enantiomers—they differ from each other in the way two mirror images differ, thanks to the asymmetrical arrangement of other atoms around a carbon atom. Chemists call one of the pair the R-enantiomer, the other the S-enantiomer.
The commercial sample I had sniffed was a fifty-fifty mix of the two enantiomers. If there is to be any hope for our hypothesis, only one of the two compounds smells like coconut, the other like porcupine, and the coconut smell overwhelms the porcupine. At least there is a well-known chemical precedent for different-smelling enantiomers. Carvone, for instance, another ten-carbon molecule, also exists in two enantiomeric forms, each with its own distinguishing odor: R-carvone is the pungent fragrance of spearmint, whereas S-carvone gives caraway its characteristic aroma.
So which of the two delta-decalactones has the smell of porcupine? To answer the question, we need two things: a specialized gas-chromatography column, known as a chiral column, that can separate optical enantiomers from each other, and a sample of authentic R- or S-delta-decalactone. Locke piques the interest of an arm of Sigma-Aldrich Co., a technology firm in St. Louis, Missouri, in the project, and the company donates three of its Supelco chiral columns to the laboratory. Guang investigates chemical databases to find a chemist who has worked with delta-decalactones, the Berlin Technical University. The stuff could all evaporate, so we don’t dare sniff it.
Now Guang performs the critical experiment. He runs the commercial mixture of delta-decalactone through the chiral column and, seventy-seven minutes after the process begins, two peaks emerge in the area of the readout where delta-decalactone appears, fifty seconds apart. When Guang spikes the commercial mixture with the S-enantiomer, the first peak enlarges. When he runs only the porcupine sample through the chiral column, a single delta-decalactone peak emerges, coinciding with the second peak of the commercial mixture. The delta-decalactone from the porcupine is therefore R-delta-decalactone. Guang and I shake hands. The project is finished.
|
What did we learn by assigning a chemical name to an odor we already recognized? We learned something about its uniqueness. The porcupine has a strong interest in sending an unambiguous message. A message that says “porcupine here” is preferable to one that says “perhaps porcupine here, perhaps something else.” If a predator has ever had a prior painful encounter with a porcupine, the unique odor would be more likely to trigger the impulse to retreat.
A chemical name is also a specific entry into the large dictionary of natural odors. Smells make up a rich natural language for most mammals, playing a key role in social structure, navigation, and much else. But people for the most part are insensible to the variation and meaning of smells, both for our species and others. At present, the best tool for learning the rudiments of such languages is chemistry.
Chemistry will also help decipher other mysteries of the porcupine, including the fluorescent characteristics of porcupine quills—yet another mechanism of warning off nocturnal predators. That may be a mystery I leave for another scientist. To him or to her, I can offer a very good pair of used vinyl gloves.
![]()
 |
Copyright © Natural History Magazine, Inc., 2006